Host Cell Protein Testing Market Pricing Analysis and Competitive Landscape (2025-2032)
Host Cell Protein Testing Market Size:
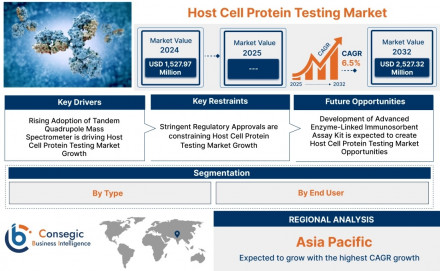
Host cell protein testing market size is estimated to reach over USD 2,527.32 Million by 2032 from a value of USD 1,527.97 Million in 2024, growing at a CAGR of 6.5% from 2025 to 2032.
Host Cell Protein Testing Market Overview:
The global Host Cell Protein Testing Market has been growing steadily, driven by increasing demand for biopharmaceuticals and stricter regulatory scrutiny. The market includes various testing technologies such as ELISA (enzyme-linked immunosorbent assay), Western blotting, and mass spectrometry. ELISA remains the most widely adopted method due to its sensitivity and scalability. The market is characterized by a mix of in-house testing solutions and outsourced services offered by contract research and manufacturing organizations. With advancements in biologics and biosimilars, especially in therapeutic areas like oncology, autoimmune disorders, and infectious diseases, the need for comprehensive HCP testing has intensified.
Host Cell Protein Testing Market Includes Drivers, Restraints & Opportunities
Key Drivers:
- Rise in Biopharmaceutical Production: The increasing production of monoclonal antibodies, vaccines, and therapeutic proteins necessitates stringent HCP monitoring.
- Regulatory Compliance: Regulatory bodies like the US FDA and European Medicines Agency (EMA) enforce strict guidelines on impurity profiling, fostering demand for advanced testing solutions.
- Technological Advancements: Innovations in HCP detection such as high-resolution mass spectrometry and multi-analyte ELISA kits improve sensitivity and specificity.
- Outsourcing Trends: Biopharmaceutical firms are increasingly outsourcing analytical testing to specialized CROs and CMOs, creating a demand for standardized and validated HCP testing services.
Key Restraints:
- High Cost of Equipment and Testing: Advanced analytical techniques require significant capital investment and skilled personnel.
- Complexity of Sample Matrices: Biologics present complex matrices that make HCP extraction and detection challenging, especially in early-phase development.
- Limited Standardization: Variability in assay development, particularly for product-specific ELISA kits, can affect reproducibility and comparability of results.
- Lack of Universal Guidelines: While regulatory frameworks exist, variations between regions and lack of harmonized standards can complicate global regulatory submissions.
Future Opportunities:
- Emergence of Biosimilars: As biosimilar approvals rise, the need for comparative HCP testing increases to demonstrate equivalency.
- Personalized Medicine and Cell Therapy: Expansion into cell and gene therapy introduces novel production systems and new HCP testing challenges.
- Development of Multiplex Assays: There is growing interest in multiplex testing platforms that can detect multiple HCPs simultaneously, improving efficiency.
- Growth in Emerging Markets: Increasing biomanufacturing activities in countries like India, China, and Brazil provide untapped potential for market expansion.
Host Cell Protein Testing Market Competitive Landscape Analysis (Key Players)
- Thermo Fisher Scientific (United States)
- Bio-Rad Laboratories, Inc. (United States)
- Cisbio Bioassays (France)
- Cygnus Technologies LLC (United States)
- Charles River Laboratories (United States)
- Agilent Technologies, Inc. (United States)
- Abcam plc. (United Kingdom)
- Cytiva (United States)
- Enzo Life Sciences Inc. (United States)
- Biogenes GmbH (Germany)
Host Cell Protein Testing Market Industry Segmentation:
By Type
- PCR-Based Assays
- ELISA-Based Assays
- Mass Spectrometry-Based Assays
- Liquid Chromatography–Mass Spectrometry (LC–MS)
- Tandem Mass Spectrometry (MS/MS)
- Others
- Others
By End-User
- Contract Research Organizations
- Biopharmaceutical Companies
- Others
By Region
- Asia-Pacific
- Europe
- North America
- Latin America
- Middle East & Africa
Regional Analysis of the Host Cell Protein Testing Market:
- North America: Dominates the market due to its advanced biopharmaceutical industry, strong regulatory oversight, and presence of key players.
- Europe: Follows closely with established biomanufacturing hubs and a supportive regulatory environment for biosimilars.
- Asia-Pacific: Expected to witness the highest growth rate due to increasing investments in biopharma R&D, rising outsourcing, and expanding clinical trials.
- Latin America and Middle East & Africa: These regions are emerging as low-cost manufacturing bases but are still developing in terms of regulatory infrastructure and technological adoption.
Host Cell Protein Testing Market Recent Developments:
- 2024 – Cygnus Technologies launched a new line of enhanced sensitivity ELISA kits to support emerging cell therapy products.
- 2023 – Thermo Fisher Scientific introduced an updated Orbitrap mass spectrometer for detailed HCP profiling in complex biologic formulations.
- 2023 – Charles River Laboratories expanded its biologics testing capabilities in Europe to meet growing demand from regional clients.
- 2022 – Bio-Rad Laboratories acquired new IP assets for product-specific HCP assays to improve assay customization and validation.
Contact us:
Consegic Business intelligence Pvt Ltd.
Contact no: (US) (505) 715-4344
Email: sales@consegicbusinessintelligence.com

